The discovery of green fluorescent protein (GFP) in the early 1960s ultimately heralded a new era in cell biology. GFP can be tagged to subcellular structures, and the readout of its fluorescence emission with an optical microscope enables to monitor cellular processes in living organisms (click here for an image gallery). Although optical microscopy based on GFP fluorescence is a powerful technique, it is usually limited due to diffraction and photobleaching. To overcome these drawbacks requires a new generation of fluorescent proteins. Recently, the GFP variant asFP595 has been isolated from the sea anemone Anemonia sulcata (Fig. 1), and its structure and spectroscopic properties have been determined in the groups of Markus Wahl and Stefan Hell at the MPI Göttingen. The asFP595 protein is an 'optical highlighter', i.e., its fluorescence emission can be selectively switched ON and OFF in response to irradiation of a particular wavelength. These favourable photochromic properties not only allow direct tracking of the movement of objects in living cells, but also open the way for a systematic improvement of the spatial resolution of optical microscopes, because fluorescence emission from regions that are out of the focus of the microscope can be selectively depleted. In a proof-of-principle experiment, the Hell group has demonstrated that, using asFP595, spatial resolution can be improved to only 50 nm (0.00005 mm) in the focal plane, which is far below the Abbe resolution (PNAS 2005, 102, 17565 ). |

|
|||
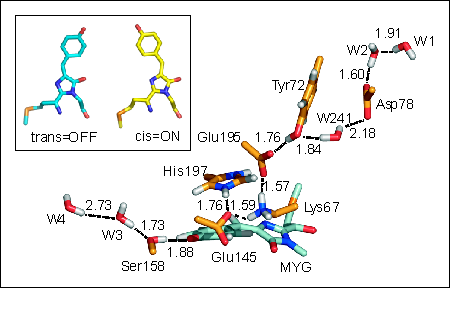
Currently, however, due to its low efficiency (quantum yield < 0.1 %) and rather slow photoswitching kinetics, the photochromic properties of asFP595 still leave much room for improvements. To systematically exploit the high potential of such switchable proteins and to enable rational improvement of its properties, e.g., by means of protein engineering, a detailed molecular understanding of the asFP595 switching mechanism is mandatory. To this end, we have elucidated the switching mechanism at the atomic level. Classical molecular dynamics simulations revealed that asFP595 photoswitching involves a trans-cis isomerisation of the chromophore molecule according to a space-saving 'hula-twist' mechanism (Fig. 2, inset). Additional mixed quantum/classical (QM/MM) calculations showed that the isomerisation triggers proton transfer events between the chromophore and adjacent amino acids (Fig. 2). This detailed understanding of the molecular mechanism underlying asFP595 photoswitching provides the first comprehensive picture of molecular photoswitching of proteins and enabled us to rationalize results from site directed mutation studies as well as to suggest new mutants with improved properties (PNAS 2005, 102, 13071 (pdf) and Angew. Chemie int. Ed., 2007, 46, 530-536 available online). |
|
|||