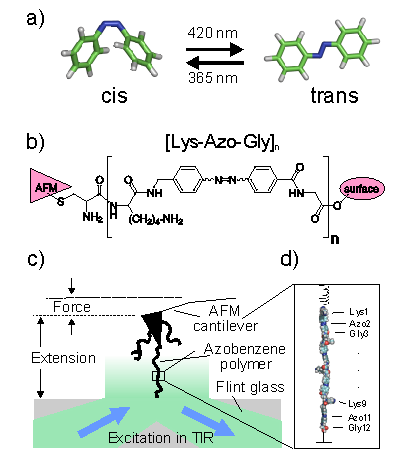
Nanomechanical devices or molecular machines will, for a broad range of applications, most likely be powered by light or other kinds of electromagnetic radiation. The major reasons are ease of addressability, picosecond reaction times to external stimuli, and compatibility with a broad range of ambient substances, such as solvents, electrolytes, or gases. Azobenzene is a wellstudied photoactive system, which can be selectively photoswitched between an extended trans and a more compact cis conformation. The trans-to-cis isomerization can be triggered by optical excitation at 365 nm, whereas the reverse cis-to-trans transition is induced by 420 nm light (Fig. 1a). Recently, Hermann Gaub and coworkers synthesized a bistable polyazobenzene peptide as a model system for a light-powered molecular machine, and characterized its mechanical properties by means of single-molecule atomic force microscope (AFM) experiments (Fig. 1b, c). Optical switching of the azobenzene polymers between their extended trans and compact cis conformations was demonstrated, and the corresponding change in the contour length of the polymer was detected. Thereby, in analogy to an Otto cycle, Gaub and coworkers established an opto-mechanical operating cycle, in which optical contraction against an external force delivered net mechanical work, thus demonstrating that, indeed, azobenzene polymers hold great promise for future applications in nanotechnology, e.g., as light-triggered molecular switches or cargo-lifters. However, the measured overall length change upon switching between the cis and trans polymers — and thus the work-output — was considerably smaller than could be expected from the length change of a single azobenzene monomer and the number of individual monomers. To resolve this discrepancy, and, more generally, to obtain a detailed microscopic understanding of the underlying polymer mechanics at the atomistic scale as prerequisite for the efficient and targeted optimization of photoswitchable polymers for future applications in nanotechnology, we carried out force-probe molecular dynamics (FPMD) simulations of polyazobenzene model peptides under mechanical stress that closely resemble the AFM experiments (Fig. 1c, d). By closely comparing to the AFM experiments, our simulations explain the observed elastic properties in terms of the underlying structural dynamics at the atomic scale. In particular, we found that the aminoacids interlinking the azobenzene units partly compensate the optical contraction and lengthening, thereby diminishing the obtainable work output. Our simulations of a polymer with stiffer linkers have shown an increased work output of about 20 %, opening the way towards the rational design of photoswitchable polymers (Angew. Chem. int. Ed., 2007, 46, 2232-2237, available online). |
|
|||