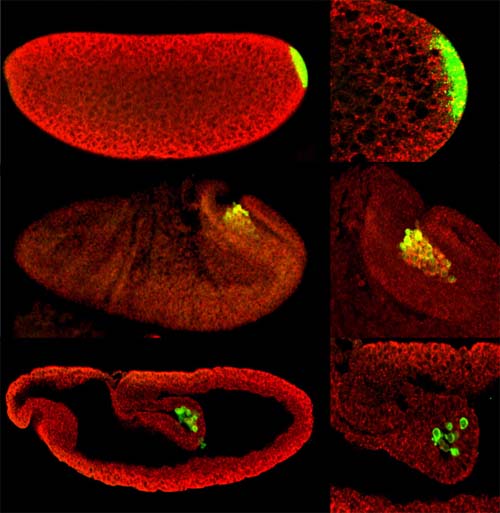
Primordial
germ cell migration
Germ cells are the only cells in the body that have the ability to form a new generation. These immortal cells are separated from the somatic cells very early during embryonic development and kept in an undifferentiated stage for a long period before their differentiation into sperm and oocytes. In many organisms, including Drosophila, primordial germ cells (PGCs) are formed in specific region early in embryonic development, which is enriched in maternally loaded RNA and proteins, named the germ plasm. In Drosophila the PGCs are the first cells to form, separated from the early syncytial somatic tissue. However, in most animals the PGCs are determined at a different position than their final destination, the gonad. The PGCs have to migrate a long distance to finally meat the zygotic gonadal precursor cells to form the embryonic gonad. In Drosophila, this cell migration starts as a passive movement with the underlying midgut anlage towards the interior of the embryo. At this timepoint the PGCs starts their active migration, the PGCs traverse the midgut epithelium, migrate dorsally towards the mesoderm, split into two cell groups and finally meat the mesoderm derived zygotic gonadal precursor cells to form two gonads laterally on both sides of the embryo. During this journey about half of the PGCs are lost and it is critically that these stem cells that are not embedded into their specific niche are removed as these PGCs can form juvenile cancer.
PGCs (green) are formed at the posterior pole and travel
with midgut into the embryo
Surprisingly, both the directed growth as well as the regulation of the survival of the PGCs is controlled by an unusual signalling system that is based on the presence of phosphorylated lipids as the key regulator of both processes, wunen, encodes a lipid posphate phosphatase (LPP). The second independent attractive signal is presumable a short geranylated peptide, however in both cases only the synthesizing or the degrading enzymes are identified but not the ligands by themselves. We became interested in the PGC migration control by two mutants we identified during our gain-of-function screen that affect both mesodermal differentiation and PGC migration. Currently, we characterize the function of these novel activities in PGC migration and muscle differentiation. |