Heparansulfate proteoglycans (HSPGs)
Heparan sulfate proteoglycans (HSPGs) are secreted or cell associated ECM proteins that are modified with specific linear heparan sulfate (HS) glycosaminoglycan polymers. Studies of mutants with impaired HS synthesis and of HSPGs themselves have revealed their essential role in the transport and reception of secreted factors, including Wingless, Hedgehog, Decapentaplegic, fibroblast growth factor and Slit. Drosophila contains four HSPGs: Perlecan, Division abnormally delayed (Dally), Dally-like protein (Dlp), and Syndecan (Sdc).
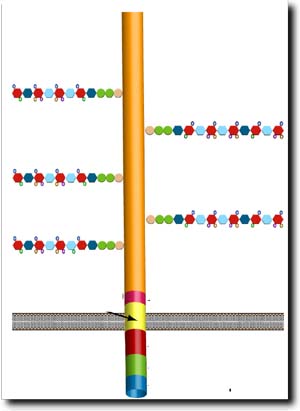
Schematic representation Sdc, including the five heparan sulfate sugar site chains
The requirement for Sdc has been established for vertebrates, showing
that the HSPG acts as an independent signaling receptor and has a number
of functional features assigned to its cytoplasmic and extracellular
domains, respectively. Its cytoplasmic domain functions in intracellular
signal transduction and plays a role in the maintenance of epithelial
integrity by linking the ECM to the actin cytoskeleton. Furthermore,
the extracellular domain of vertebrate Sdc is proteolytically shed and
acts as an extracellular effector in cell communication events.
In Drosophila, Sdc was shown to regulate Slit signaling. Slit,
a secreted ligand produced in ventral midline cells, acts as a repellent
in both axon and myotube guidance during embryogenesis, two processes
that are mediated by Robo receptors in the target cells. Loss of Slit
signaling causes axons and muscle fibers to cross the ventral midline
of the embryo, a mutant phenotype that is also observed in the absence
of Sdc activity.
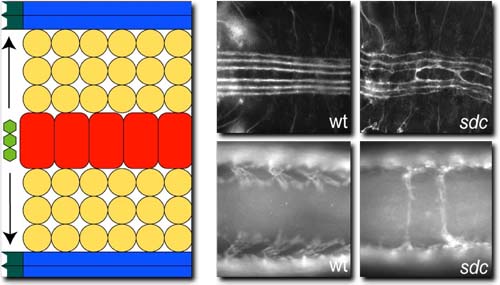
Slit is secreted from the ventral midline (red) and is required to repell a subset of axons (top) and muscles from crossing the midline. In sdc mutant embryos both axons and muscles receive a reduced amount of Slit and a subset cross the midline (right)
The analysis of the mechanisms underlying Sdc function in Slit-mediated
signaling revealed that Sdc acts in a cell-autonomous manner in Slit-receiving
cells. Surprisingly, the highly conserved cytoplasmic domain of Sdc
is not essential for this function, however its extracellular domain
is sufficient to mediate Slit signaling when it is membrane anchored.
Furthermore, Drosophila Sdc activity can be replaced by the
human homolog hsdc2, although the essential extracellular domain of
the two Sdc proteins are not conserved except for their heparan sulfate
and chondroitin sulfate modifications suggesting the sugar modifications
are essential for Slit binding. However, since the mutation of all heparan
sulfate modification sites in Sdc had no effect on its activity we propose
that the chondroitin sulfate modifications of Sdc mediate the specific
binding of Slit. This argument is further supported by the finding that
the membrane associated HSPG Dally-like protein (Dlp) can only partially
substitute for Sdc function, although it is heavily modified with heparan
sulfate. Based on these results we suggest a model in which the heparan
sulfate modifications of Dally and Dally–like enable their function
in Hh, Wg and FGF interaction whereas the chondroitin sulfate modification
of Sdc are required for its specific function for Slit signaling. Furthermore,
the requirement of Sdc in the receiving cells and the lack of importance
of the cytoplasmic domain are in accordance with a specific function
of Sdc as presenting co-receptor of Slit for its receptor Robo.
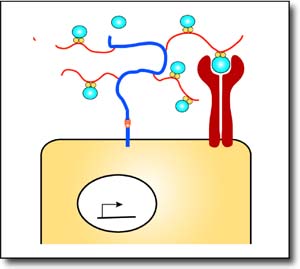
Sdc functions on the Slit receiving cells as a co-receptor presenting Slit to its receptor Robo via its chondroitin sulfate modifications