
Next: Acknowledgements
Up: Molecular Dynamics of Conformational
Previous: A Markov Model
We studied the conformational dynamics of a simplified
protein model by molecular dynamics simulations
at long time scales up to 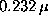 s.
The model has been designed as a minimal model, intended to include
only those structural elements, which can be assumed to be
essential for the low-frequency dynamical properties of proteins.
As shown by our simulations the model actually exhibits
properties similar to those of more realistic protein models,
such as tertiary structure, vibrational spectra,
a hierarchy of time scales, and the occurrence of rare transitions
between conformational substates.
s.
The model has been designed as a minimal model, intended to include
only those structural elements, which can be assumed to be
essential for the low-frequency dynamical properties of proteins.
As shown by our simulations the model actually exhibits
properties similar to those of more realistic protein models,
such as tertiary structure, vibrational spectra,
a hierarchy of time scales, and the occurrence of rare transitions
between conformational substates.
Two effective descriptions for the conformational dynamics of the model,
which both neglect memory effects, were
compared with an explicit MD-simulation. To enable an analysis, a rigorous
theoretical concept of conformational substates based on the notion of
free energy landscapes has been formulated and applied.
The first model, a Langevin model, describing the
dynamics within a certain conformational state at a picosecond
time scale, could not reproduce conformational transition rates derived
from MD-simulations. This failure was found to be due to
memory effects, caused by correlations between many degrees of freedom, which
strongly influence the short time scale dynamics within conformational states
at the picosecond time scale.
The second description, a Markov model, aimed at an analysis of the protein
dynamics at the much longer time scale of few hundred picoseconds.
Here, the analysis of the distribution of conformational
transition times suggested, that
the conformational dynamics of our model
does not exhibit memory effects.
These findings demonstrate a qualitative change in the dynamical
behavior of our model protein, when proceeding from the short
time scales, which are at present accessible by MD-simulations
of realistic protein models, to longer time scales:
whereas memory effects play a significant role at short time scales,
they appear to vanish at longer time scales. As a result the
slower conformational dynamics can be described by a master equation.
Care has to be taken in the attempt to generalize these results to the
dynamical behavior of real proteins.
As argued in the first part of Section  , we expect the
polymer chain of our simple model to be much more flexible than that of
real proteins. Correspondingly, their conformational dynamics
--- as far as collective motions of the polypeptide chain are involved ---
should occur at slower time scales.
For this reason, one can not conclude, that
memory effects in protein dynamics are actually absent
at that hundred picosecond
time scale characteristic for conformational transitions of
our model. However, the results do
suggest that memory effects in the dynamics of proteins
generally tend to vanish at long time scales.
Furthermore, because enhanced rigidity of real proteins,
the time scale of few hundred picosecond
can be considered as a lower bound for the absence of memory effects.
, we expect the
polymer chain of our simple model to be much more flexible than that of
real proteins. Correspondingly, their conformational dynamics
--- as far as collective motions of the polypeptide chain are involved ---
should occur at slower time scales.
For this reason, one can not conclude, that
memory effects in protein dynamics are actually absent
at that hundred picosecond
time scale characteristic for conformational transitions of
our model. However, the results do
suggest that memory effects in the dynamics of proteins
generally tend to vanish at long time scales.
Furthermore, because enhanced rigidity of real proteins,
the time scale of few hundred picosecond
can be considered as a lower bound for the absence of memory effects.


Next: Acknowledgements
Up: Molecular Dynamics of Conformational
Previous: A Markov Model
Helmut Grubmueller
Mon Nov 6 16:25:56 MET 1995
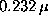 s.
The model has been designed as a minimal model, intended to include
only those structural elements, which can be assumed to be
essential for the low-frequency dynamical properties of proteins.
As shown by our simulations the model actually exhibits
properties similar to those of more realistic protein models,
such as tertiary structure, vibrational spectra,
a hierarchy of time scales, and the occurrence of rare transitions
between conformational substates.
s.
The model has been designed as a minimal model, intended to include
only those structural elements, which can be assumed to be
essential for the low-frequency dynamical properties of proteins.
As shown by our simulations the model actually exhibits
properties similar to those of more realistic protein models,
such as tertiary structure, vibrational spectra,
a hierarchy of time scales, and the occurrence of rare transitions
between conformational substates.