Muscle adhesion
Morphological aspects of somatic muscle and heart formation as well as
the underlying gene activities are conserved between vertebrates and Drosophila.
Each muscle fiber originates from a distinct muscle founder cell that
is determined by specific combinations of transcription factors. Subsequently,
the founder cells form syncitial myotubes by the fusion with specific
competent mesodermal cells. In parallel, the syncitial myotube extend
by the formation of polar growth cone-like structures at their polar ends.
As a result, myotubes elongate and extend along the epidermis towards
their apodemes at the segment borders. The approaching myotube secretes
Vein, an EGF that accomplishes the final differentiation of those apodemes
that are directly contacted by myotubes. After contacting each other,
myotubes and apodemes start their final differentiation, a process characterized
by the expression of proteins of the contractile apparatus.
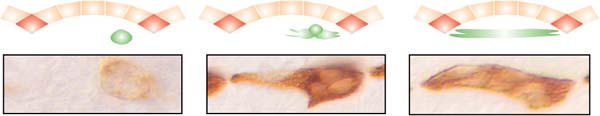
Each muscle originate from a single muscle founder (left), fuses with myoblasts (center) and grow towards its later attachment sites within the epidermis (right)
The anchorage of the muscles is assured by integrin proteins. Integrins
are heterodimeric proteins which in Drosophila consists of specific
a-subunits that dimerise with a common b-subunit
in most of the tissues. In case of the muscle apodeme anchorage the apodemes
and muscle express the complementary set of the so called “position
specific” PS-Integrins. However, although this complementary set
of PS-Integrins is required for the attachment between many different
cell types the Integrin proteins do not interact directly with each other
in the intercellular space. Instead, the Integrin dimers interact with
specific proteins of the extra cellular matrix (ECM). We have shown that
the muscle-specific Integrins interact with Thrombospondin, which is expressed
and secreted from the apodeme cells. This interaction is one of three
characterized interactions that are required in a combinatory way to finally
anchor the muscles at the epidermal apodemes. However, based on the strength
of the phenotype, Thrombospondin seems to be the most important anchoring
partners of the Integrins at the apodemes. Furthermore, we could show
that Thrombospondin expression is controlled early in parallel to stripe
via competing Wingless and Hedgehog signaling but late in the differentiated
apodeme cells directly by Stripe activity. Therefore, Thrombospondin is
one of the genes that are directly regulated downstream of stripe
being essential for muscle within the apodemes.
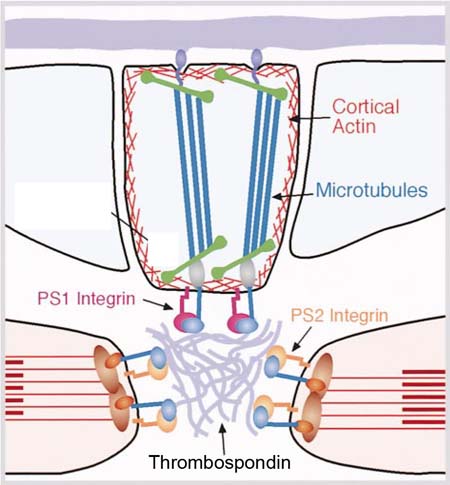
Each
muscle is attached to epidermal apodemes sites via Integrins bound to
the ECM-protein Thrombospondin
secreted from the apodemes
To withstand the contracting forces of the muscles, the apodemes are characterized
by an intensive network of cytoskeleton that transfer the forces of the
muscles through the apodeme cells towards the cuticule that is stably
attached towards the basal site of the apodemes. Therefore, many genes
required for muscle anchorage encode proteins of this highly complex cytoplasmic
network.



