Muscle screens
Mutant studies suggest, that the directed extension of the myotubes is controlled by signalling processes between the prospective apodemes and the extending myotubes. Only a few genes and two signal transduction pathways were shown to be involved in the control of the directed outgrowth of myotubes: (i) the transcription factor encoded by the stripe gene which is both necessary and sufficient for the differentiation of the apodemes. Its activity regulates a number of downstream genes required for the directed extension of myotubes towards apodemes, (ii) Slit/Robo signalling which is initially required for the repulsion of muscle progenitors from the ventral midline region and later for the guidance of myotube towards the apodemes and (iii) FGF signalling which is necessary for the dorsal outgrowth of the mesoderm by activation the mesodermal FGF-receptor Heartles. Furthermore, the approaching myotube secretes Vein, an EGF that accomplishes the final differentiation of those apodemes that are directly contacted by myotubes.
We performed a gain-of-function screen to identify genes required for muscle attraction and repulsion. The basis of this screen is a library of about 12.000 so called EP-elements that we generated in a combined afford of the EMBL and the company Develogen. The EP-elements are randomly integrated genetic elements that carry a GAL4-dependent promotor that enables the directed expression of neighboring genes, if the EP-element is inserted upstream of the gene and in the correct orientation. This type of gain-of-function screen allows the screening for gene activities that upon ectopic expression can function in specific biological circuits resulting in a phenotype.
The screen was performed using a GAL4-driver line that was based on a mutant variant of the stripe promotor that enables expression within the apodeme precusor cells at the segment border with the idea to screen for gene activities that can function as inhibitors of the directed myotube growth and their adhesion towards the expressing apodemes later. In parallel the promotor directed expression in an ectopic domain, a stripe of cells positioned anteriorly to the differentiating apodemes at the segment border. This additional expression allowed to screen in parallel for gene activities with the potential to function as attractants resulting in an ectopically attached myotubes (see figure below).
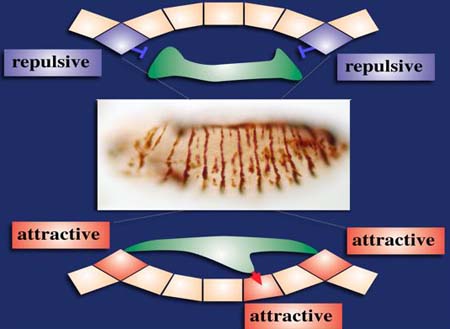
Gain-of-function screen that allows the screening for repulsing and attracting activities in parallel.
Among
4,500 genes tested for the induction of lethality caused by the ectopic
expression, we identified more than 70 genes that cause a lethal muscle
pattern defect, including syndecan (sdc). Although the
majority of genes identified are not expressed or at least not specifically
expressed within the developing apodemes we identified a subgroup of candidates
that like Sdc are specifically expressed within the segmentborder cells
or even more specific only within the apodeme cells.
To prove that the ectopic expression is the cause of a muscle pattern
defect we analyzed the muscle patterns. In the example shown in the figure
below, ectopic expression within the muscles marked in red that normally
never contact the apodeme cells at the segment border, resulted in an
ectopic migration and adhesion at the segment border cells. These specific
muscles became responsive to the guiding cues from the segment border
upon ectopic expression.

The muscles marked in red do not attach to the apodemes at the segment borders in the wild type (left), however expression of one gene within the muscles renders them to become sensitive to the guidance cues originating from the segment borders.



